340B Transparency Efforts Uncovering More Drug Company Overcharges
by Admin | December 8, 2021 4:19 pm
Dec. 8, 2021– In the nearly three years since the Health Resources & Services Administration (HRSA) launched its 340B drug pricing program ceiling price website, 41 drug companies have acknowledged that they overcharged 340B hospitals, health centers, and clinics and would refund those affected. This contrasts with only seven such reports in the two years prior to the launch in April 2019.
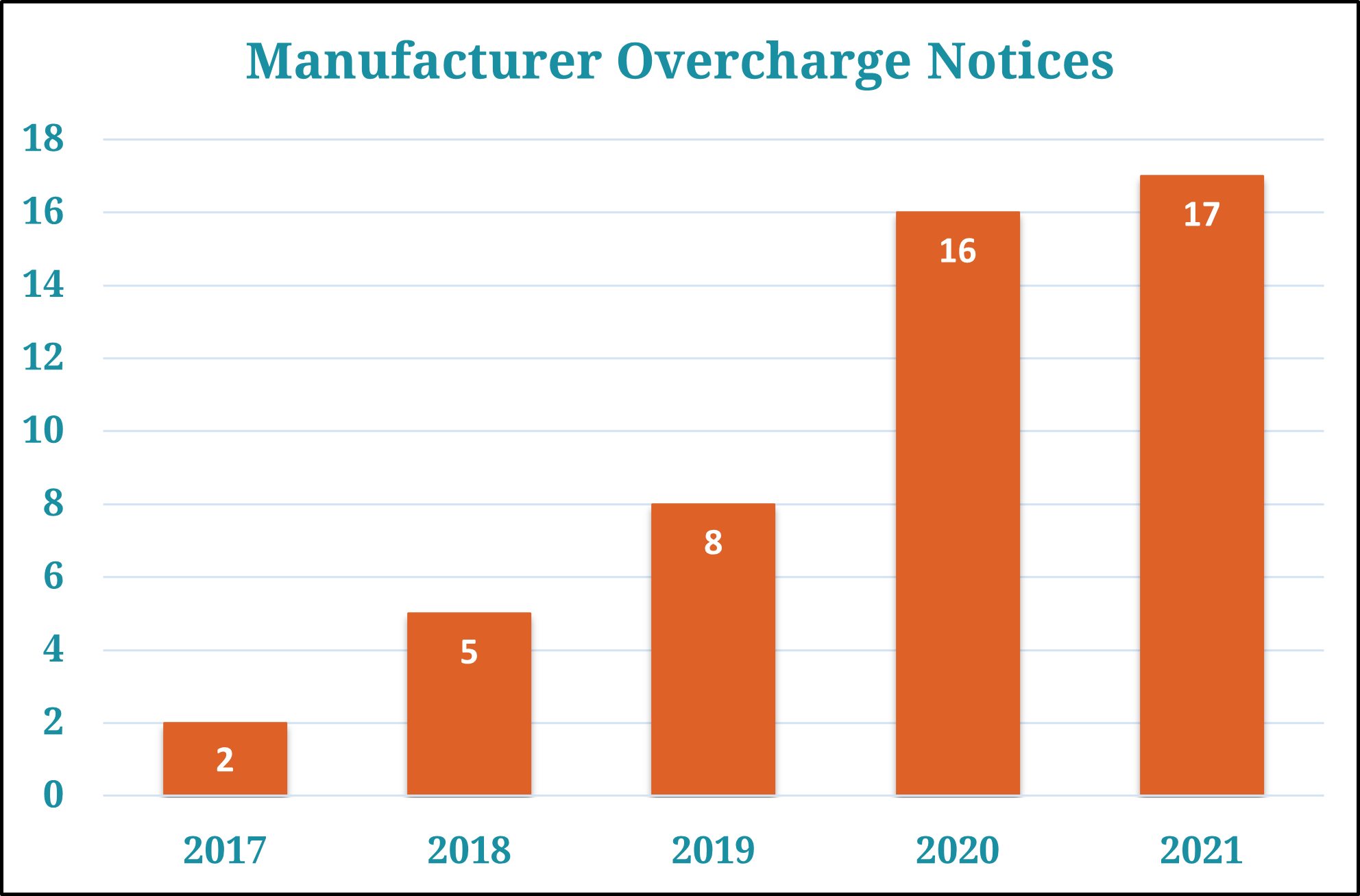
The ceiling price website lists the maximum amounts that 340B covered entities can be charged for eligible drugs under the program. 340B Health and other advocates for the website argued that the addition of such pricing transparency would lead to better compliance with the law by the more than 600 drug makers that participate in 340B. Drug companies submitting quarterly pricing data for the site who determine that their prices exceeded the maximums are directed to post notices of the overcharges on HRSA’s 340B website[1] to notify providers that they may be eligible for refunds. In 2019, drugmakers posted eight such notices. In 2020, that figure jumped to 16 and as 2021 ends, companies have posted a record 17 overcharge notices. Prior to the launch of the ceiling price website, drugmakers posted only five overcharge notices in 2018 and two in 2017.
In recent years, HRSA also has been conducting five annual audits of drug companies to determine their compliance with 340B law, a transparency process that is in addition to the voluntary overcharge postings. For fiscal year 2021, which ended on Sept. 30, HRSA found evidence of overcharging by four of the five companies it audited. That also marks an all-time high. From FY 2015 to FY 2018, HRSA found such evidence in slightly more than 6% of its manufacturer audits. Since FY 2019, however, that average has jumped to nearly 67%.
Let the Sun Shine
The sharp rise in overcharge findings appears to be linked directly to the creation of the ceiling price website. Legislation enacted in 2010 authorized the website following the release of a series of reports by the Department of Health and Human Services (HHS) Office of Inspector General (OIG) identifying drugmaker overcharges. A 2003 report[2], for example, concluded that five manufacturers of 11 prescription drugs overcharged 340B-covered entities an estimated $6.1 million for the drugs over a year. “The overcharges represented 45% of the amount paid by the covered entities,” the report stated.
The legislation called for HRSA to develop a secure website that would allow manufacturers and providers to review data establishing the ceiling price for each drug eligible for 340B discounts. Development of the website was delayed during the Obama and Trump administrations, but following a lawsuit brought by 340B Health and allied associations, HRSA launched it on April 1, 2019. Starting in January 2019, HRSA began collecting quarterly drug pricing data from drug companies participating in the 340B program to populate the website.
Covered entities can consult the ceiling price website to determine whether they are paying the correct prices. If they determine they are paying too much, they can follow up with distributors or manufacturers and file overcharge reports[3] with HRSA if they cannot resolve the issues that way. In addition, the quarterly data collection process uncovers instances in which drug companies are charging too much before the information appears on the website.
340B hospitals and other providers benefit from this transparency. The manufacturer overcharge notices that appear on the HRSA website detail the steps that companies must take to refund providers who paid too much for 340B drugs. In the cases of audit findings involving overcharges, HRSA directs the companies to repay affected providers and orders the implementation of corrective action plans to ensure the overcharges will not happen again.
Community Pharmacy Dispute
The biggest example of drug company overcharges is the ongoing dispute over 340B discounts for drugs dispensed by community-based pharmacies. Ten drug companies have unilaterally imposed limits on 340B discounts involving such pharmacies or have announced their plans to do so. In May, HRSA sent letters to the first six of these companies informing them that they were violating the 340B statute requiring them to charge no more than the ceiling price for any eligible drug, and the agency sent a letter to a seventh company in October. All seven have gone to court to try to block HRSA from enforcing the law, and another three companies since have pursued their own pricing restrictions.
To gather information the information needed to make determinations of overcharges, HRSA relies on the reports filed by hospitals and other covered entities that have been denied the purchase of covered drugs at the 340B price. HRSA cited those reports in the notices they sent to noncompliant companies, and government attorneys have cited them in many of the briefs they have filed in the pending lawsuits.
Another section of the 2010 law established civil monetary penalties of up to nearly $6,000 for each instance of overcharging. In September, HRSA asked[4] the HHS OIG to consider imposing such fines on six companies – Eli Lilly, AstraZeneca, Novo Nordisk, Novartis, Sanofi, and United Therapeutics. If the OIG imposes such fines, the cost to these companies is likely to be in the tens or hundreds of millions of dollars.
- 340B website: https://www.hrsa.gov/opa/manufacturer-notices/index.html
- 2003 report: https://oig.hhs.gov/oas/reports/region6/60100060.pdf
- overcharge reports: https://www.340bpvp.com/Documents/Public/340B%20Tools/340B-ceiling-price-unavailable-incorrect-340b-ceiling-price-notification-for-hrsa.pdf
- asked: https://www.hrsa.gov/sites/default/files/hrsa/opa/pdf/updated-hrsa-letter-eli-lilly-covered-entities.pdf
Source URL: https://340binformed.org/2021/12/340b-transparency-efforts-uncovering-more-drug-company-overcharges/